Last Updated:
The Health Ministry will hold an emergency meeting today with state health officials to tackle the crisis of contaminated cough syrups after 14 children died in MP’s Chhindwara.

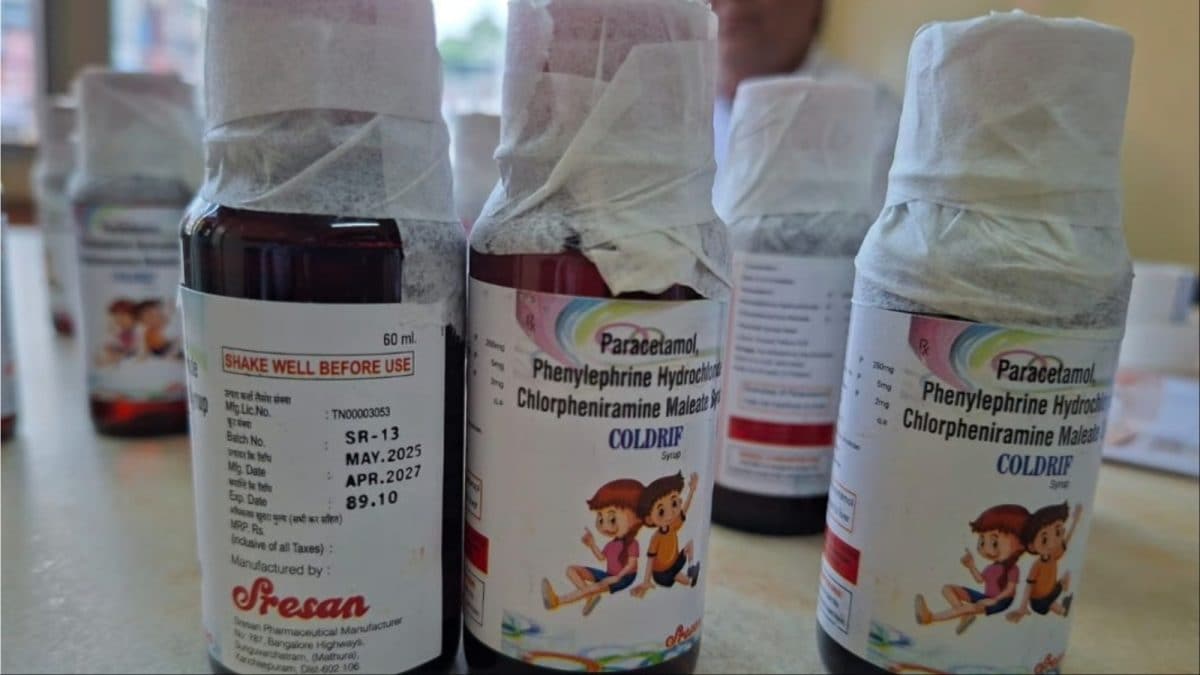
Coldrif cough syrups (Photo: Social Media)
The Ministry of Health is set to hold an urgent meeting at 4.00 pm on Sunday to address the critical issue of contaminated cough syrups following the death of multiple children in Madhya Pradesh.
The meeting, which will include Principal Secretaries, Health Secretaries, and Drug Controllers from all States and Union Territories (UTs), will be chaired by the Health Secretary and will have two focus points, the rational use of cough syrups and the quality of drugs.
Recommended Stories
According to the details, today’s meeting aims to reinforce control measures and ensure the safety and quality of pharmaceutical products in the country, especially over-the-counter medicines like cough syrups, which are generally not recommended for children under five years of age.
As many as 14 children in Madhya Pradesh’s Chhindwara have died due to suspected renal failure after they consumed the Coldrif cough syrup.
Later, officials stated that the samples of the drug were found to contain 48.6 per cent diethylene glycol, a highly toxic substance.
Subsequently, the Madhya Pradesh government on Saturday banned the sale of Coldrif cough syrup.
COUGH SYRUP SAMPLES TESTED IN MP
A test report received by the Madhya Pradesh government from the Tamil Nadu-FDA on Saturday revealed that a sample of ‘Coldrif‘ was found adulterated.
The diethylene glycol, or the DGE, found in the syrup, is an industrial chemical used in anti-freeze and brake fluids, and is known to cause acute kidney failure and death when ingested.
Following the report, the Madhya Pradesh government announced a ban on the sale of the ‘Coldrif‘ syrup and all other products from its manufacturer, Sresan Pharmaceutical, Kancheepuram, based in Tamil Nadu.
The ‘Coldrif‘ sample, which the Tamil Nadu-FDA tested to have beyond permissible limits of DEG/EG, was also sampled for analysis by the Madhya Pradesh-Drug Regulatory Authority, whose final results are awaited.
Meanwhile, background tests conducted by the Central Drugs Standard Control Organisation (CDSCO) on six other drug samples, including antibiotics, antipyretics, and ondansetron, consumed by children who fell ill in Chhindwara, were found not to contain DEG/EG contaminants.
In response to the tragedy, the Centre has taken a tough stand and is promising a massive crackdown on all drug makers found to be compromising quality.
The CDSCO has ordered risk-based inspections of drug manufacturing units across the country to identify regulatory gaps and prevent future quality failures.
The CDSCO is also pressing for strict action against the manufacturer of the cough syrup under scanner in Tamil Nadu, M/s Sresan Pharmaceutical.
DOCTOR ARRESTED IN MADHYA PRADESH
As the authorities swung into action, a doctor in Chhindwara was arrested on Sunday. Most of the deceased children were treated at the private clinic of paediatrician Dr Praveen Soni in Parasia.
Soni, who is also employed as a government doctor, reportedly prescribed the Coldrif syrup to several children suffering from cough and seasonal fever.
Following the incident, the Madhya Pradesh government also registered a case against Srisan Pharmaceuticals.
19 MEDICINES SUSPENDED IN RAJASTHAN
Meanwhile, Rajasthan Health Minister Gajendra Singh Khinvsar on Saturday said that 19 medicines supplied by Kaysan Pharma, Jaipur, have been suspended following reports of adverse effects linked to Dextromethorphan HBr Syrup in Bharatpur and Sikar.
Two health officials, Dr Palak Koolwal (PHC Hathideh) and Pharmacist Pappu Soni, were suspended for negligence, while State Drug Controller Rajaram Sharma was also suspended for lapses in monitoring.
On September 28, 2025, complaints were received from Bharatpur regarding batch number KL-25/147 of Dextromethorphan Syrup. The next day, a similar complaint came from Sikar regarding batch KL-25/148.
“Patients reported vomiting, dizziness, fainting, and drowsiness after consuming the syrup. The Health Department immediately banned these batches and sent statutory samples to the State Drug Testing Laboratory,” the minister said.
Fresh advisories from the Drug Controller General of India (DCGI) warn that dextromethorphan should not be given to children under two years of age and should generally be prescribed only for those above five years.
The Rajasthan government has instructed all doctors to strictly follow these guidelines, he added.
TAMIL NADU BANS ‘COLDRIF’ COUGH SYRUP
Following the deaths, the Tamil Nadu government has also banned the sale of cough syrup ‘Coldrif‘ and ordered its removal from the market following suspicions linking it to the death of children in Madhya Pradesh and Rajasthan.
With effect from October 1, the sale of the cough syrup manufactured by the city-based firm has been prohibited across Tamil Nadu, news agency PTI quoted an official of the Food Safety and Drug Administration Department as saying.
Inspections were conducted at the pharmaceutical company’s manufacturing facility in Sunguvarchathram in neighbouring Kancheepuram district during the last two days, and samples have been collected, he said.
The company, he said, supplies the medicines to Rajasthan, Madhya Pradesh, and Puducherry.
The samples would be sent to the government-run laboratories to test for the presence of the chemical ‘Diethylene Glycol’, he told PTI.
KERALA BANS COLDRIF COUGH SYRUP
Kerala Health Minister Veena George on Saturday said the state Drugs Control department has suspended the sale of Coldrif cough syrup in the state.
A preliminary inquiry by the State Drugs Control Department revealed that the flagged batch of the drug was not sold in Kerala, the minister clarified in a statement.
“However, the Drugs Controller, out of concern for safety, has instructed drug inspectors to completely stop the distribution and sale of Coldrif,” she said.
This drug is being sold through eight distributors in Kerala, the minister said, adding that all these centres have been instructed to halt distribution and sale.
Besides, the minister said that instructions have been issued to suspend the sale of Coldriff syrup through medical stores as well.
RAIDS IN UTTARAKHAND
On Saturday, the Uttarakhand government launched raids on medical stores and wholesale drug dealers across the state in the wake of child deaths in Madhya Pradesh and Rajasthan allegedly due to contaminated cough syrups.
Uttarakhand Health Secretary and FDA Commissioner Dr R Rajesh Kumar told reporters that drug inspectors in all districts have been ordered to collect samples of cough syrups from hospitals and shops in a phased manner within the month and have their quality laboratory tested so that any defective or harmful drugs can be immediately removed from the market.
Chief Medical Officers of the state have been ordered to implement the advisory received from the central government with immediate effect.
ALSO READ | Centre Confirms DEG Contamination In Madhya Pradesh Cough Syrup Samples Amid Probe Into Child Deaths
October 05, 2025, 09:33 IST
Loading comments…
Read More