Last Updated:
The products reported missing include insulin formulations marketed as Ryzodeg FlexTouch and Fiasp (Penfill and FlexTouch), along with various strengths of Wegovy FlexTouch.

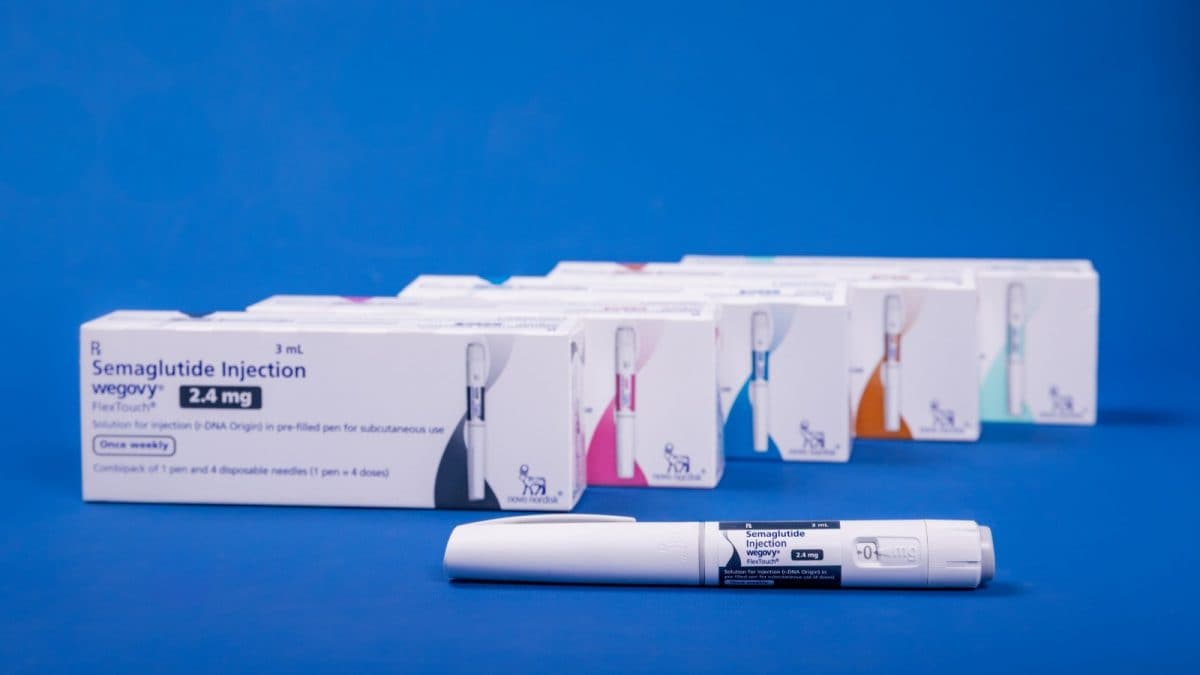
Among the stolen products is Wegovy, Novo Nordisk’s blockbuster weight-loss injection. Image/News18
India’s drug regulator has sounded an alert after Danish drugmaker Novo Nordisk reported theft of several batches of its injectable medicines, including insulin and obesity treatment drugs, during transit from the company’s Bhiwandi hub to cities such as Nagpur, Raipur, Cuttack and Kolkata, News18 learnt.
The Drugs Controller General of India (DCGI), Dr Rajeev Singh Raghuvanshi, in a notice issued to state drug controllers and regulatory offices across the country, said the stolen products may pose safety risks to patients since they require strict cold-chain storage at 2°C to 8°C. If handled outside this range, the quality and efficacy of the medicines could be compromised.
Recommended Stories
“M/s Novo Nordisk has informed about a theft of its products… The quality of the products may be compromised if the products are not handled in proper storage conditions since the formulations are supposed to be maintained at 2°C to 8°C failing which, would impact the quality of the drug and in turn impact the safety of the patients,” said the letter issued by CDSCO on 21 August, seen by News18.
The products reported missing include insulin formulations marketed as Ryzodeg FlexTouch and Fiasp (Penfill and FlexTouch), along with various strengths of semaglutide injection sold under the brand Wegovy FlexTouch. The matter is under investigation by the police.
Among the stolen products is Wegovy, Novo Nordisk’s blockbuster weight-loss injection that has seen surging global demand as an anti-obesity treatment. The drug, a GLP-1 analogue originally developed for diabetes, was launched in India in June amid rising interest in medical interventions for obesity.
News18 reached out to Novo Nordisk for a response on the theft and subsequent alert, but the company had not replied immediately. This story will be updated once a response is received.
Regulator issues advisory
Regulators have asked doctors to exercise caution while prescribing these drugs and to advise patients to promptly report any adverse reactions. “Carefully prescribe and educate the patients for reporting of any adverse drug reactions.”
Consumers have been urged to procure medicines only from authorized sources and with proper invoices. Meanwhile, officials across states have been instructed to keep a strict vigil on the movement of these products in the market and initiate action wherever necessary under the Drugs and Cosmetics Act, 1940. “…To be careful and procure the above products from authorized sources only and with proper invoice,” the letter advises.
The Central Drugs Standard Control Organisation has also directed its IT cell to publish the alert on its website to ensure wider public awareness.
“Instruct your officers to keep a strict vigil on the movement of the said products in the market and initiate necessary action under the provisions of Drugs & Cosmetics Act, 1940 and Rules made thereunder.”
About the Author

Himani Chandna, Associate Editor at CNN News18, specialises in healthcare and pharmaceuticals. With firsthand insights into India’s COVID-19 battle, she brings a seasoned perspective. She is particularly pass…Read More
Himani Chandna, Associate Editor at CNN News18, specialises in healthcare and pharmaceuticals. With firsthand insights into India’s COVID-19 battle, she brings a seasoned perspective. She is particularly pass… Read More
Loading comments…
Read More